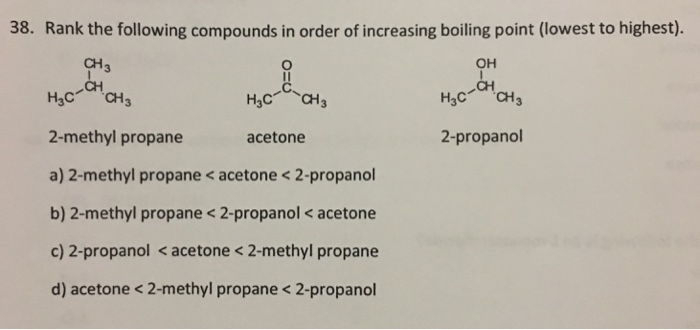
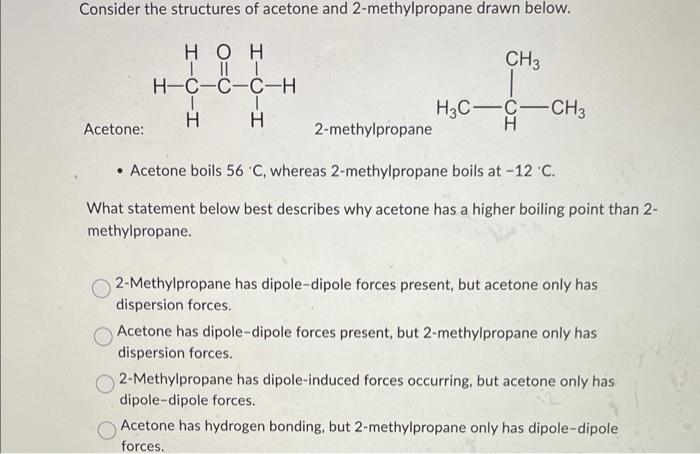
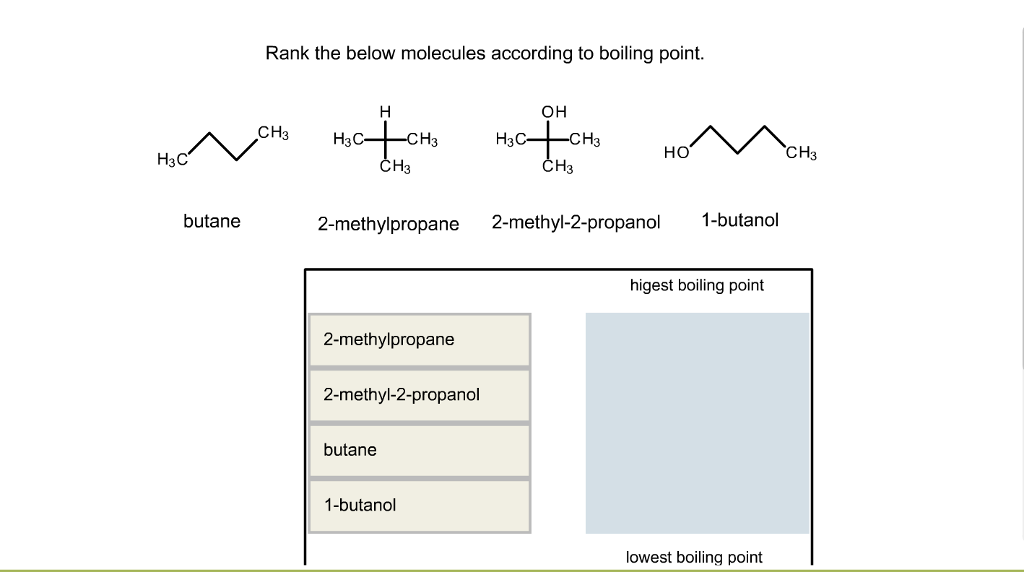
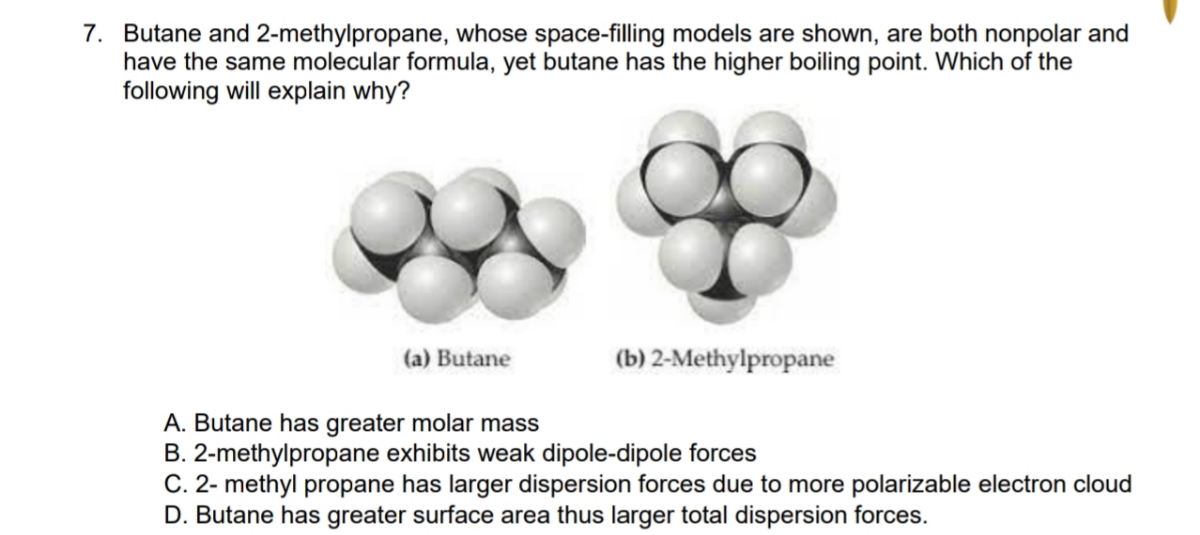
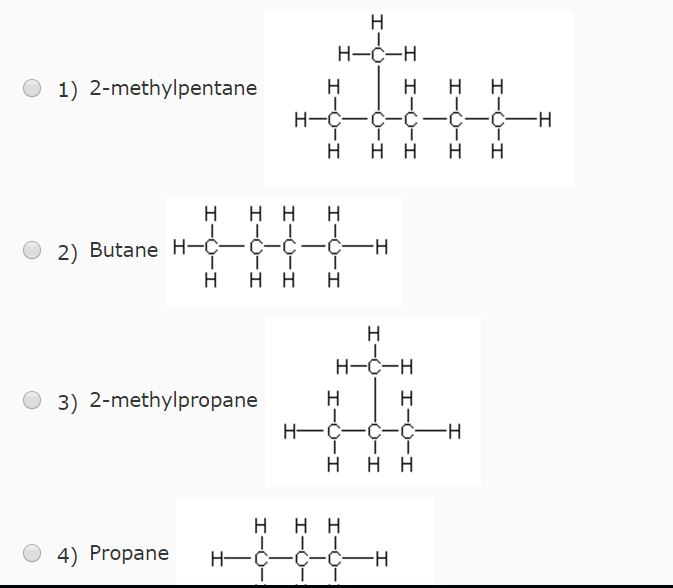
40. Lowest boiling point is for 1) butanol 2) Pentagon 3) 2 methyl propane 2 ol 4) 2 methyl butane 2 ol
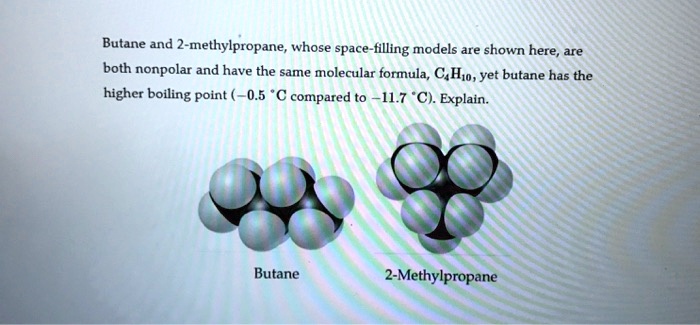
SOLVED: Butane and 2-methylpropane, whose space Hlling models are shown here are both nonpolar and have the same molecular formula C,H,o, yet butane has the higher boiling Point 0.5 C compared t0 -

SOLVED: Question 23 (a) Butane and 2-methylpropane are both non-polar and have the same molecular formula,C4H1o. Explain why butane has a boiling point of -0.5-C and 2-methylpropane has a boiling point of -
![SOLVED: Problem 11.23 Butane and 2-methylpropane whose space-filling models are shown are both nonpolar and have the same molecular formula yet butane has the higher boiling point] (0.5 C compared t0 -11.7 SOLVED: Problem 11.23 Butane and 2-methylpropane whose space-filling models are shown are both nonpolar and have the same molecular formula yet butane has the higher boiling point] (0.5 C compared t0 -11.7](https://cdn.numerade.com/ask_previews/558c9c9f-69dc-4898-b90e-97ee9014db79_large.jpg)