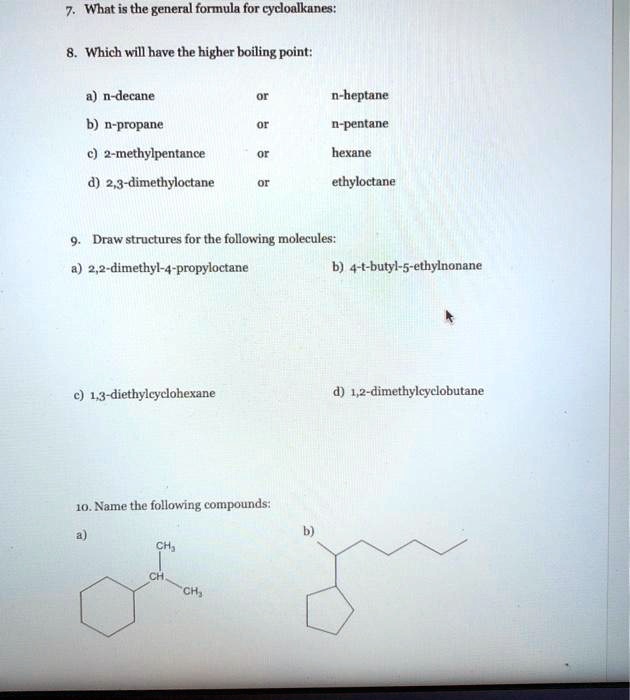
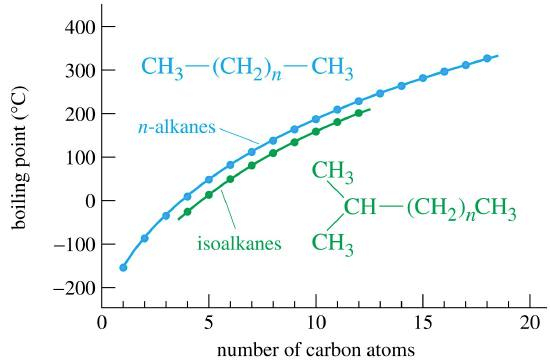
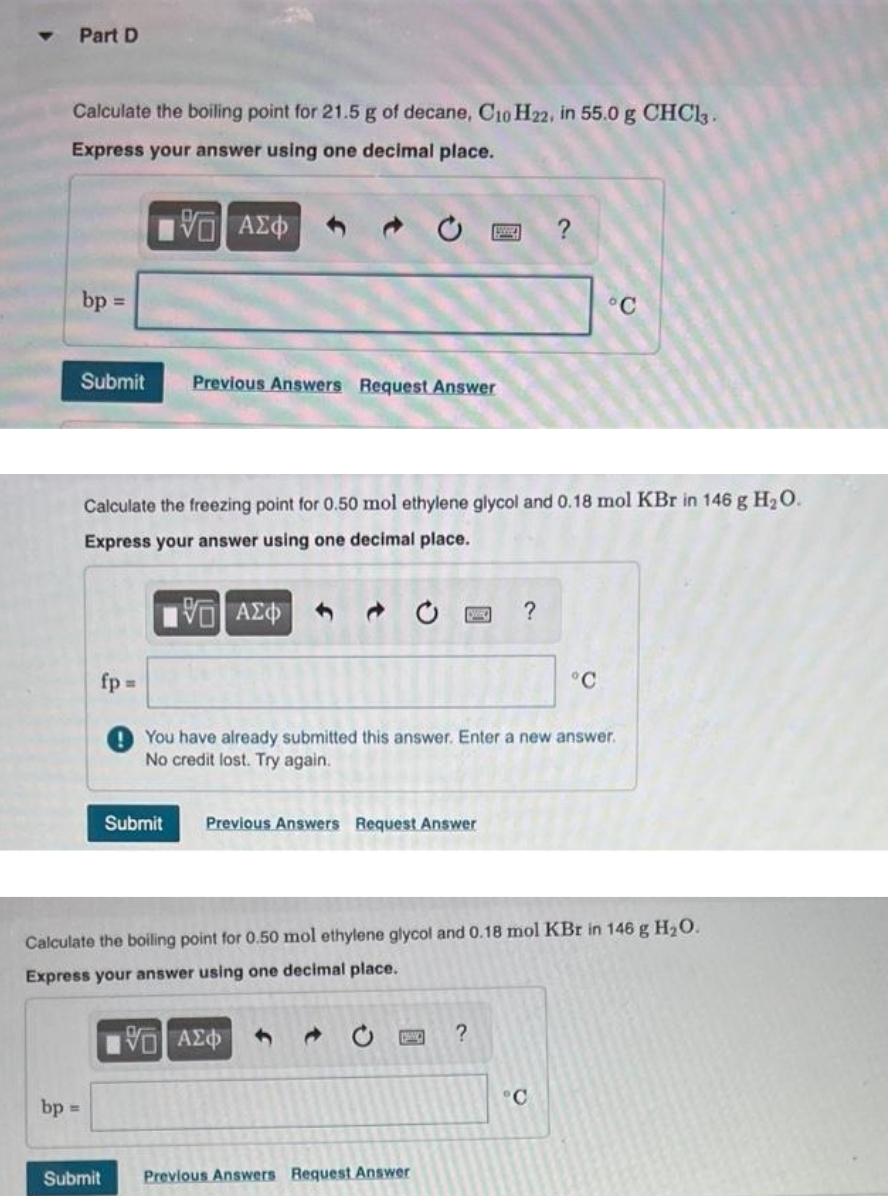
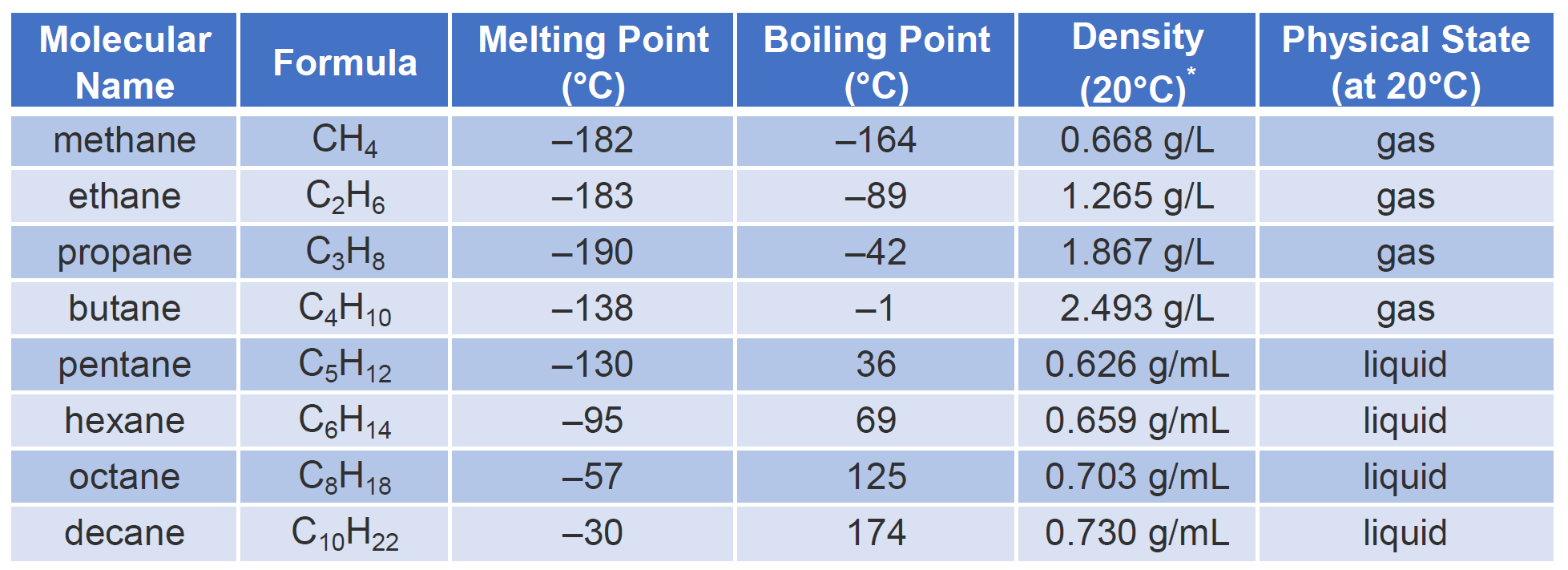
![SOLVED: Part C Estimate Ihe normal boiling point of decane using Trouton rule [estimating that 4 approximateli 87-88 J/(K mol)] assuming that Ihe enthalpy ol vaporizalion, Hyap 39.58 kJ /mol remains relatively SOLVED: Part C Estimate Ihe normal boiling point of decane using Trouton rule [estimating that 4 approximateli 87-88 J/(K mol)] assuming that Ihe enthalpy ol vaporizalion, Hyap 39.58 kJ /mol remains relatively](https://cdn.numerade.com/ask_images/db22f275da734093a6121d5332bc534c.jpg)
SOLVED: Part C Estimate Ihe normal boiling point of decane using Trouton rule [estimating that 4 approximateli 87-88 J/(K mol)] assuming that Ihe enthalpy ol vaporizalion, Hyap 39.58 kJ /mol remains relatively
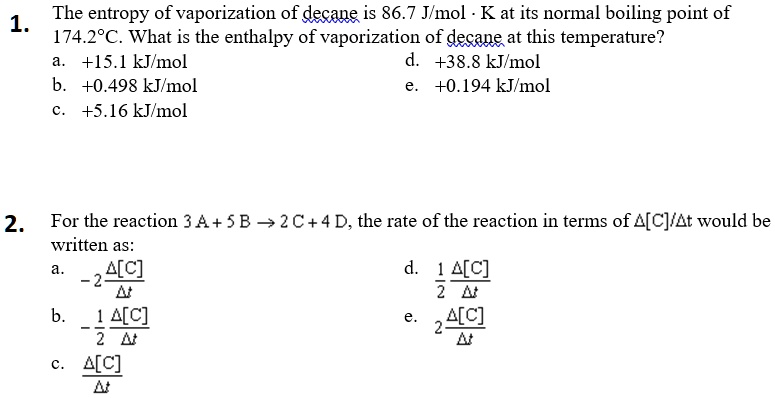
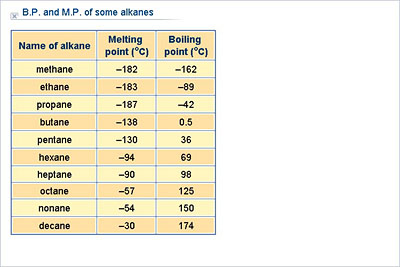
SOLVED: The entropy of vaporization of decane is 86.7 Jlmol Kat its normal boiling point of 1 174.28C . What is the enthalpy of vaporization of decane at this temperature? +15.1 kJmol +
Why does the decane (C10H22) have a higher boiling point than CH2CL2? If the decane is apolar (London forces) and ch2cl2 is polar (London and dipole-dipole) why does the hydrocarbon have a

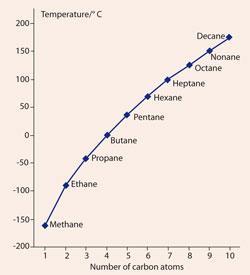
Properties of Alkanes Nonpolar molecules – not water-soluble Relatively low melting and boiling points Generally less dense than water The longer the chain. - ppt download

Liquid Viscosity and Surface Tension of n-Hexane, n-Octane, n-Decane, and n-Hexadecane up to 573 K by Surface Light Scattering | Journal of Chemical & Engineering Data