![High-Performance n-Hexane Purification by Nonporous Adaptive Crystals of Leaning Pillar[6]arene | CCS Chemistry High-Performance n-Hexane Purification by Nonporous Adaptive Crystals of Leaning Pillar[6]arene | CCS Chemistry](https://www.chinesechemsoc.org/cms/asset/15f6d039-c380-4724-9d85-6d1107da3d90/keyimage.jpg)
High-Performance n-Hexane Purification by Nonporous Adaptive Crystals of Leaning Pillar[6]arene | CCS Chemistry

Evaporation (p, T ) diagram for liquid n-hexane. The solid line is for... | Download Scientific Diagram
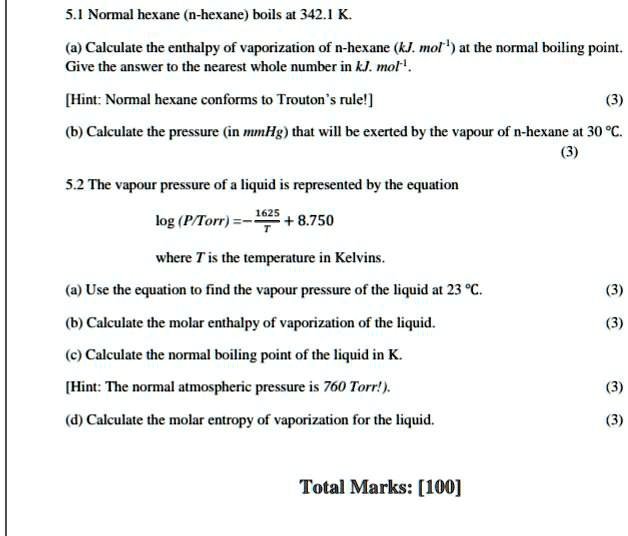
SOLVED: 5.1 Normal hexane (n-hexane) boils at 342.1 K (a) Calculate the enthalpy of vaporization of n-hexane (kI mol 1 at the normal boiling Point, Give the answer [0 the nearest whole

Viscosity of n-hexane as a function of temperature for selected pressures. | Download Scientific Diagram

SOLVED: IU n-Hexane boils at 68.7 'C at 1.013 bar; with a molar enthalpy of vaporization of kJ/mol. What is the change in 28.85 the molar entropy (J/Kmol) of n- hexane when