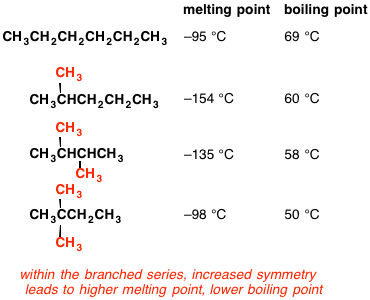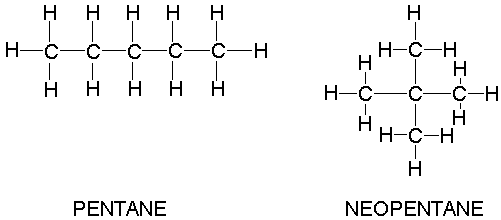
Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase? | Socratic
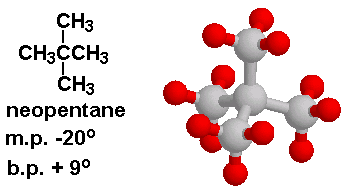
Why is the melting point of neopentane higher than n-pentane but the melting point of isopentane lower than that of n-pentane? Why is the order different than that of their boiling points? -

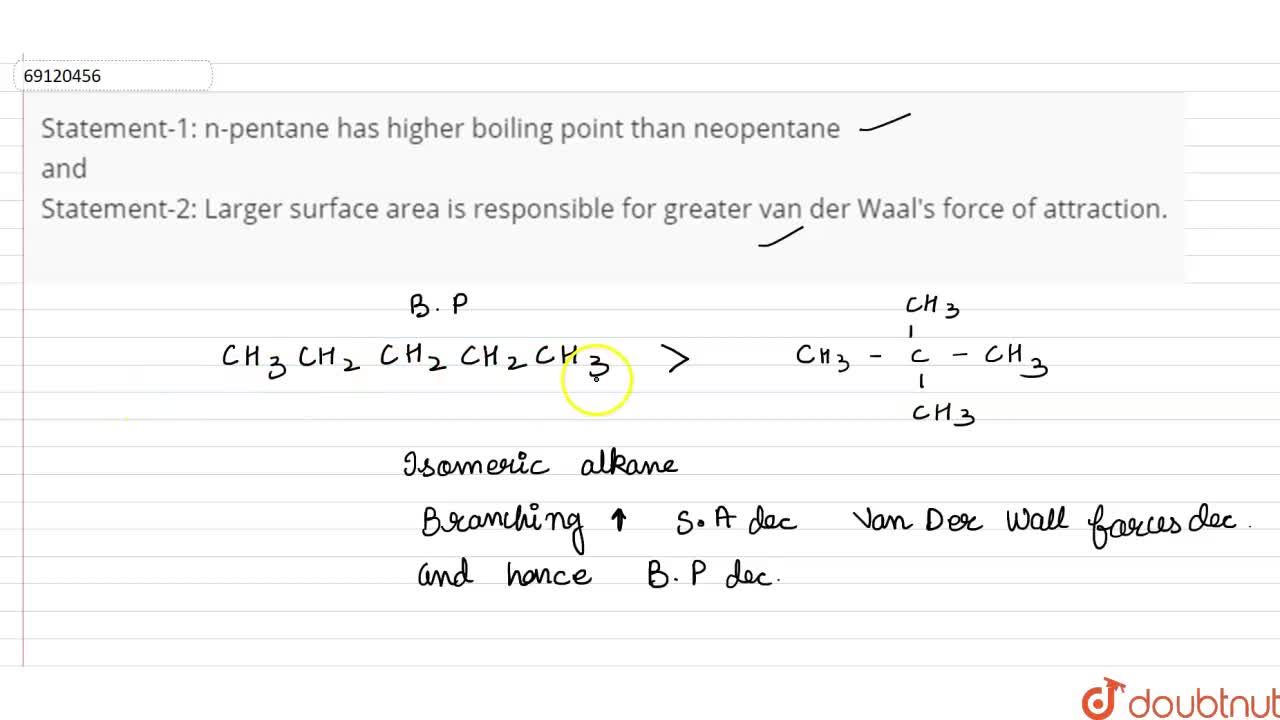
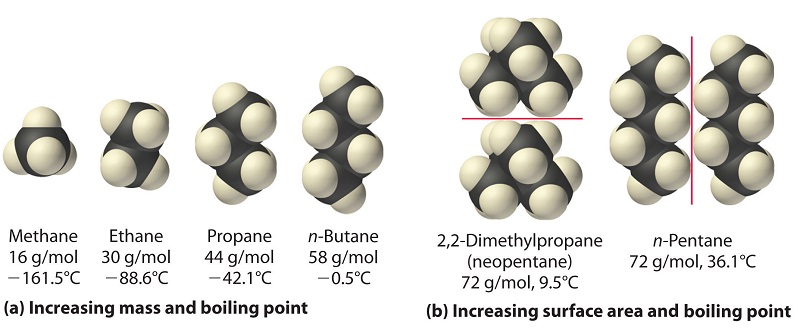
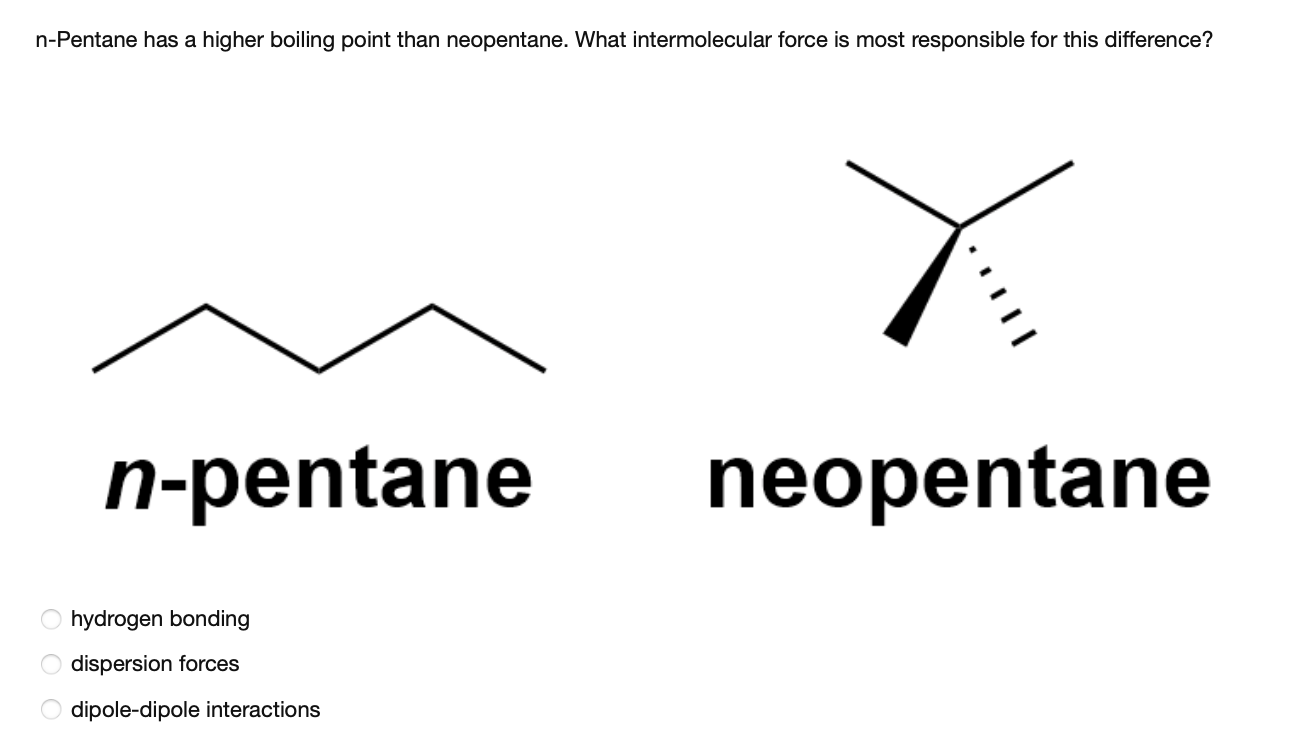
Arrange the following in decreasing order of their boiling points.(I) n - Butane (II) 2 - Methylbutane(III) n - Pentane(IV) 2,2 - Dimethylpropane

Pentane has a boiling point of 36.1 degrees Celsius while 1-butanol, which has a similar mass, has a boiling point of 117.7 degrees Celsius. Explain this difference, including line-angle structures of each

Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com
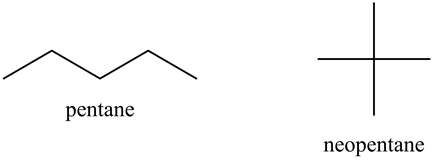
Rank these compounds by boiling point from highest to lowest boiling point: pentane, neopentane, hexane - Home Work Help - Learn CBSE Forum
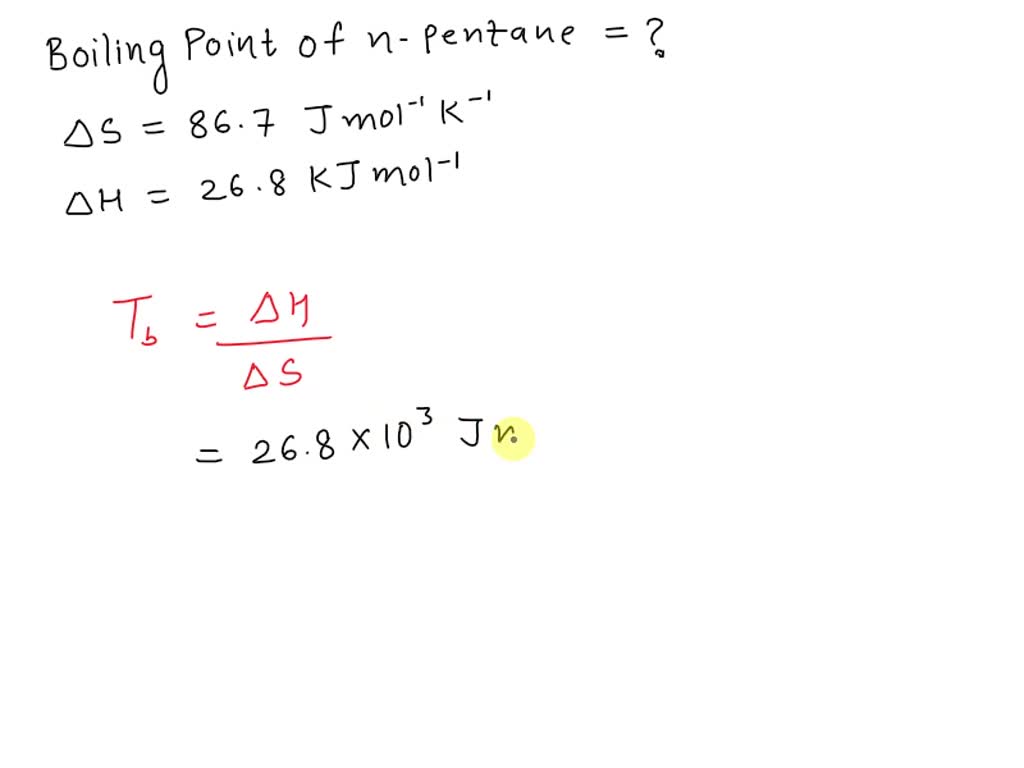
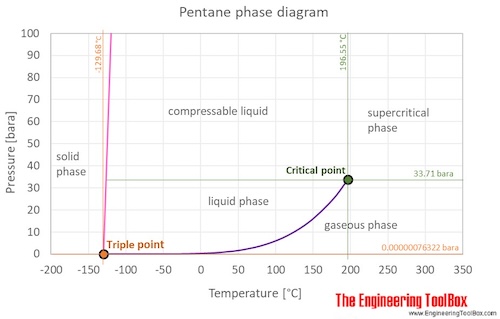
SOLVED: Calculate the boiling point (BP) of n-pentane, given that the = Points) entropy 14)(6= BPis86.7 Jlmol x *K and the change in enthalpy is 26.8 kJlmole change at the
Why Isomers of a compound have different Boiling point (like Isomers of pentane) why force of attraction is not involve in it? - Quora