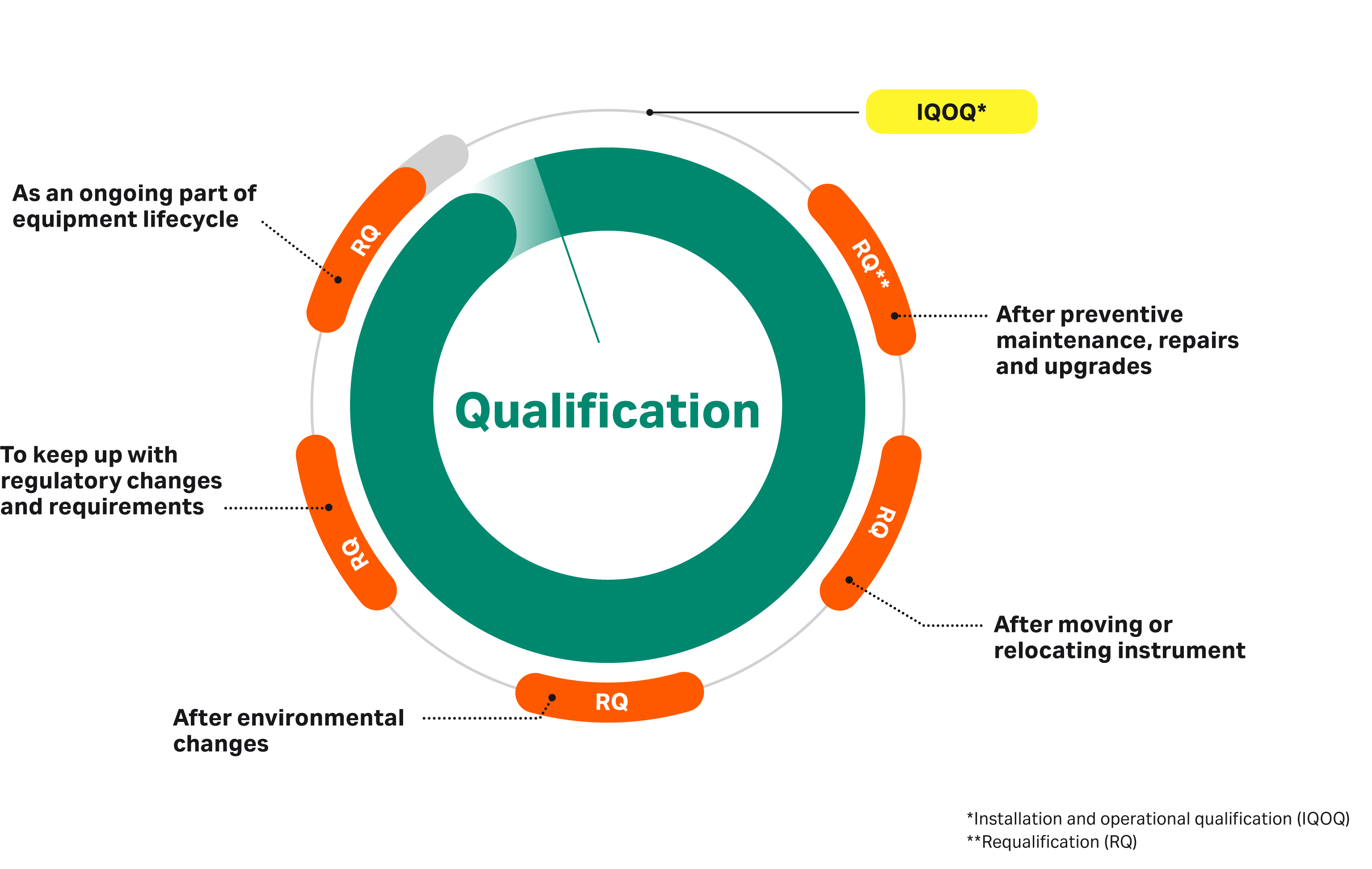
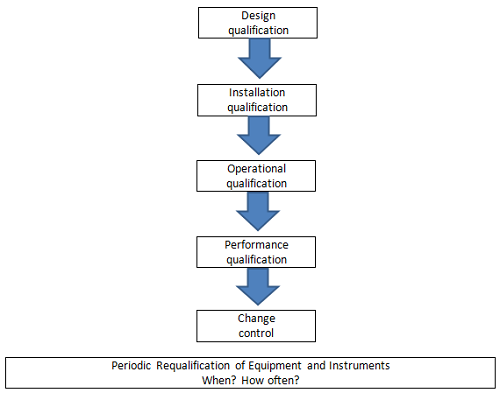
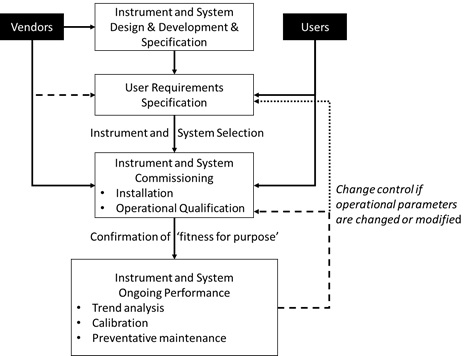
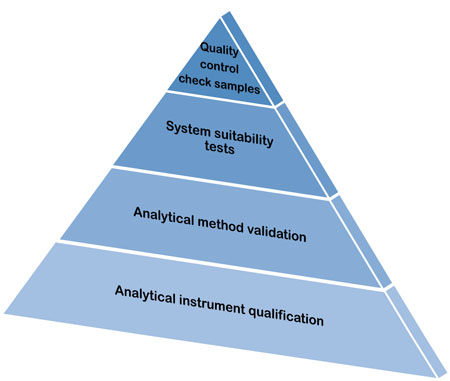
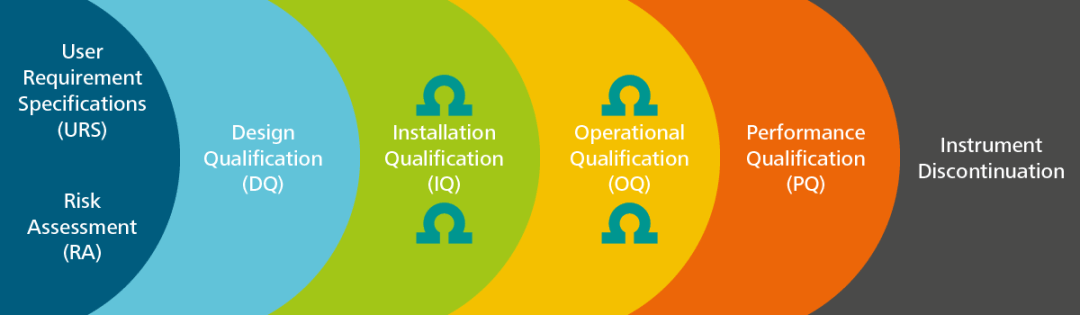
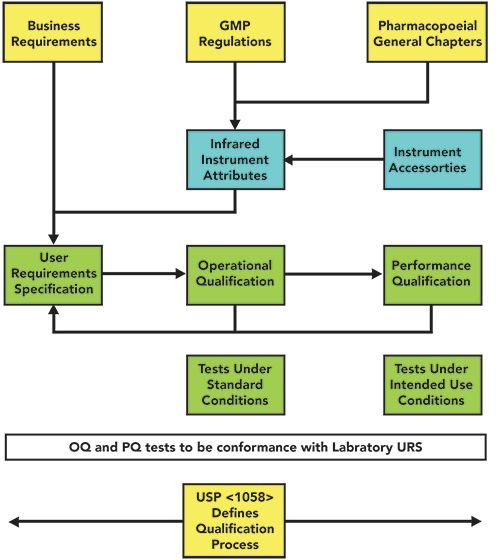
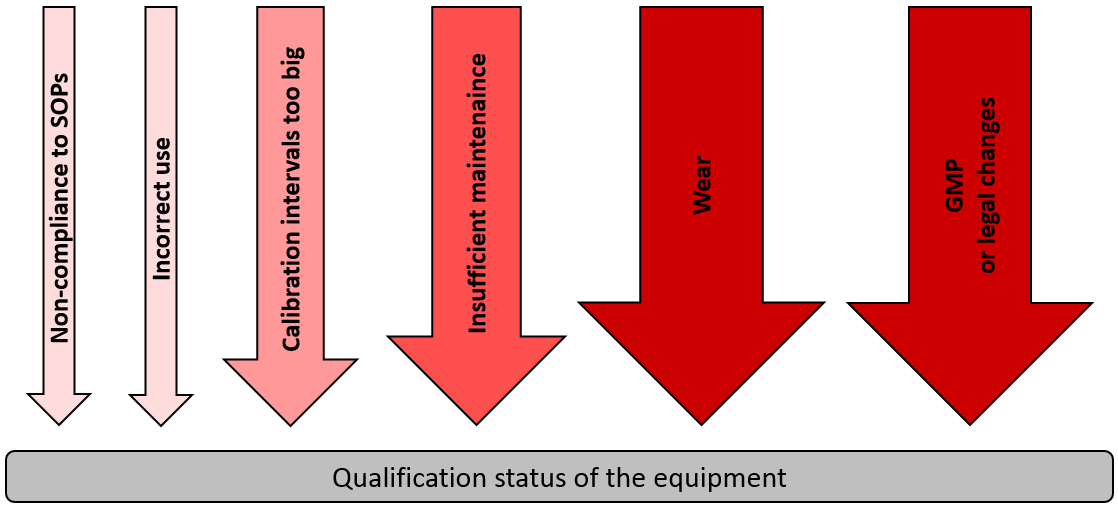
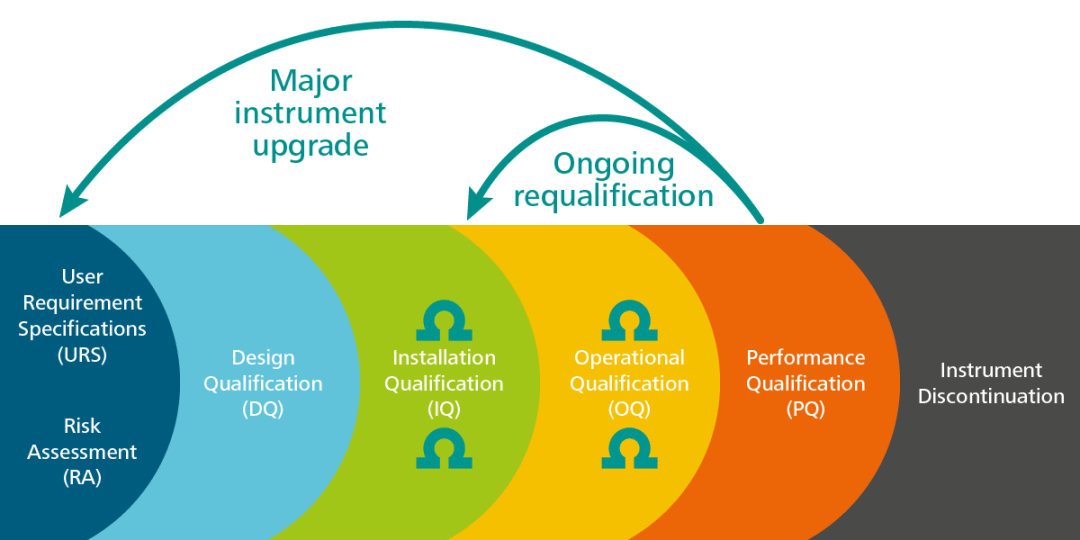
PDF) Qualification of analytical instruments for use in the pharmaceutical industry: A scientific approach

Analytical instrument qualification in capillary electrophoresis - Cianciulli - 2012 - ELECTROPHORESIS - Wiley Online Library

Practical Approaches to Method Validation and Essential Instrument Qualification: 9780470121948: Medicine & Health Science Books @ Amazon.com
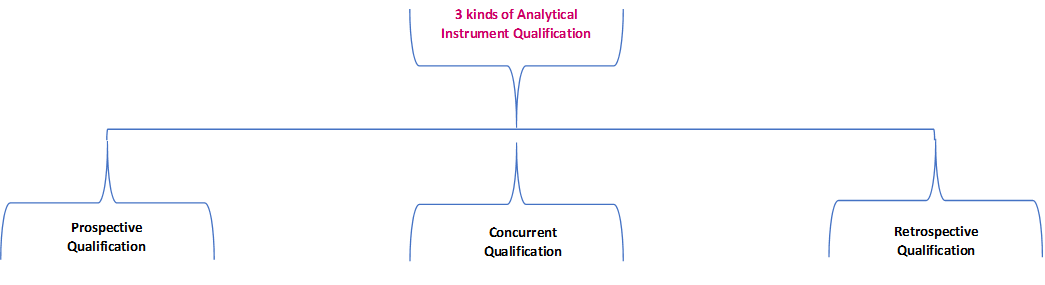
![PDF] AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF PHARMACEUTICAL INDUSTRY | Semantic Scholar PDF] AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF PHARMACEUTICAL INDUSTRY | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/81d9dc060c2784957b63ed435a8b472b79e3af08/2-Figure1-1.png)