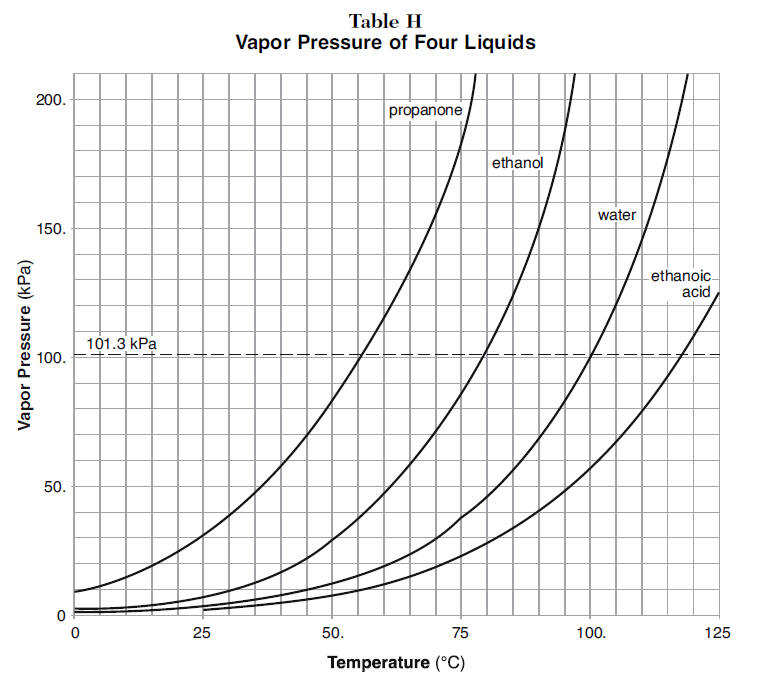
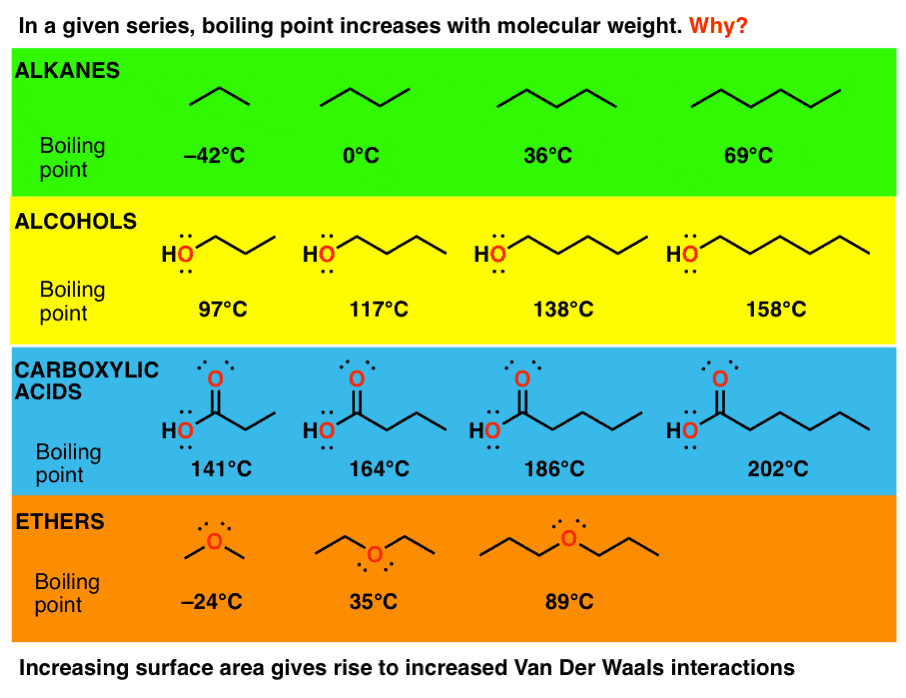
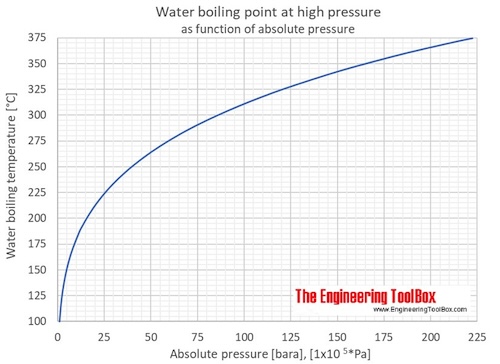
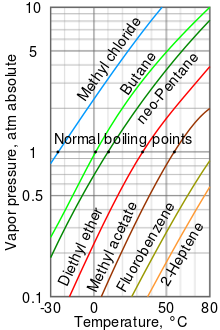
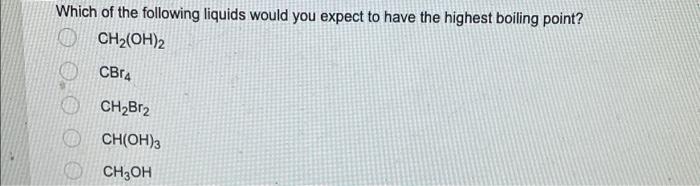
Distillation is a widely used method for separating mixtures based on differences in boiling points as they - Brainly.com

Entropy | Free Full-Text | Large Eddy Simulation and Thermodynamic Design of the Organic Rankine Cycle Based on Butane Working Fluid and the High- Boiling-Point Phenyl Naphthalene Liquid Heating System
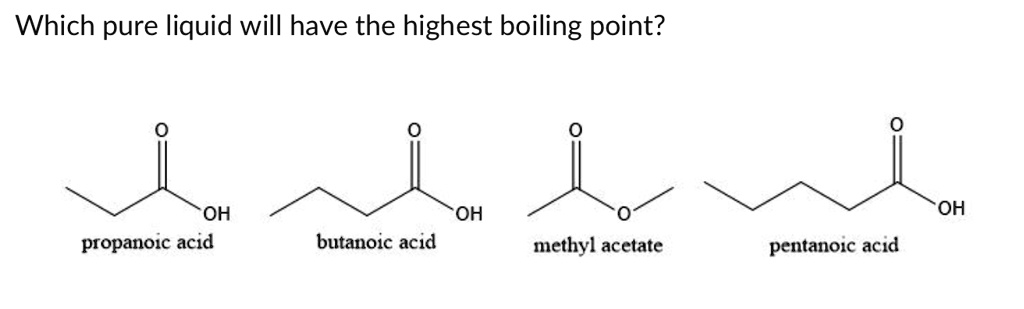
SOLVED: Which pure liquid will have the highest boiling point? OH propanoic acid OH butanoic acid OH methyl acetate pentanoic acid
its normal boiling point is -189°C. Oxygen is a gas at room temperature. If the normal melting point of a substance is below room temperature, the substance is a liquid at room temperature. Benzene melts at 6°C and boils at 80°C; it is a liquid at room ...