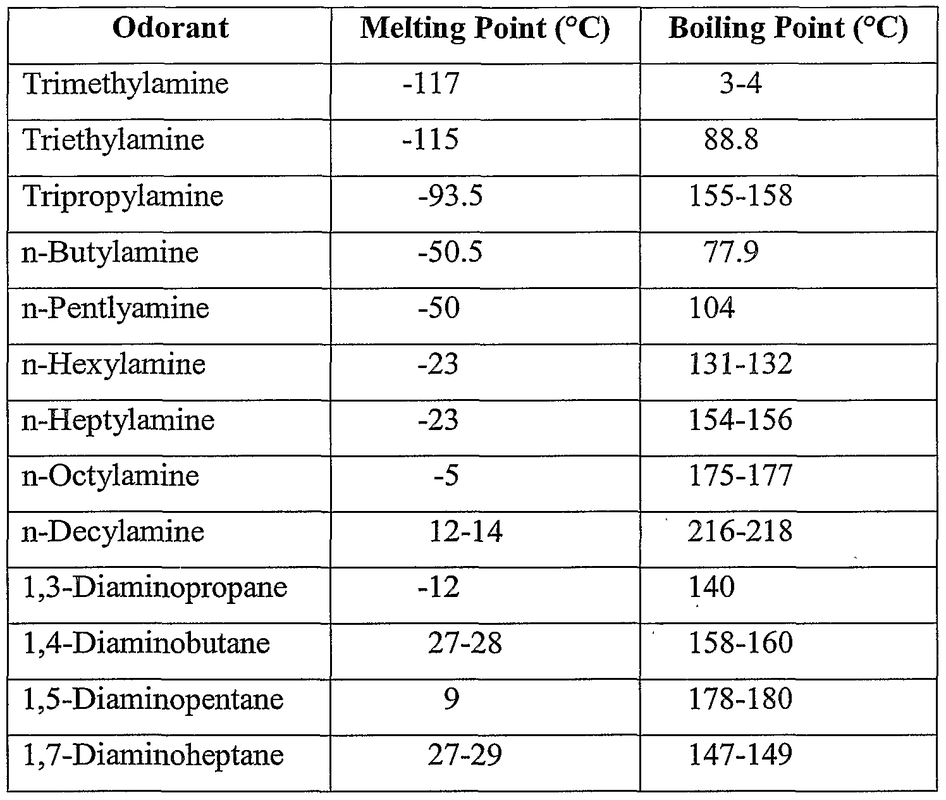
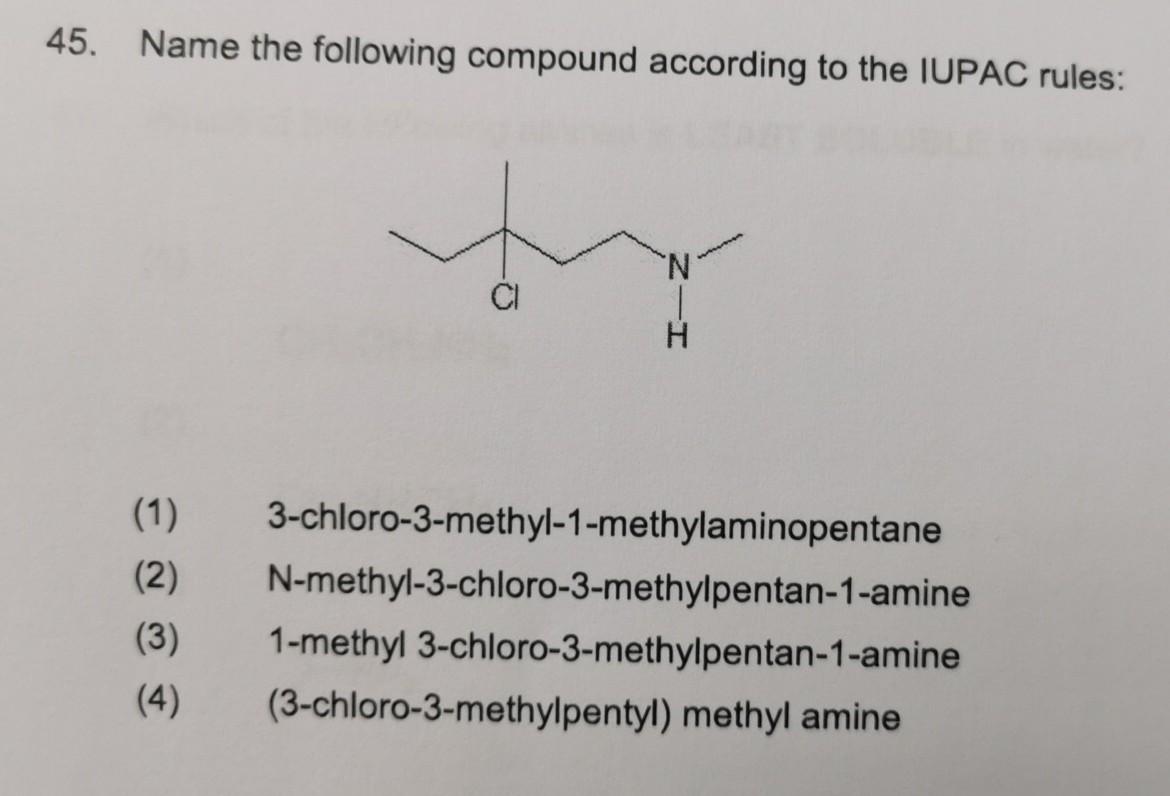
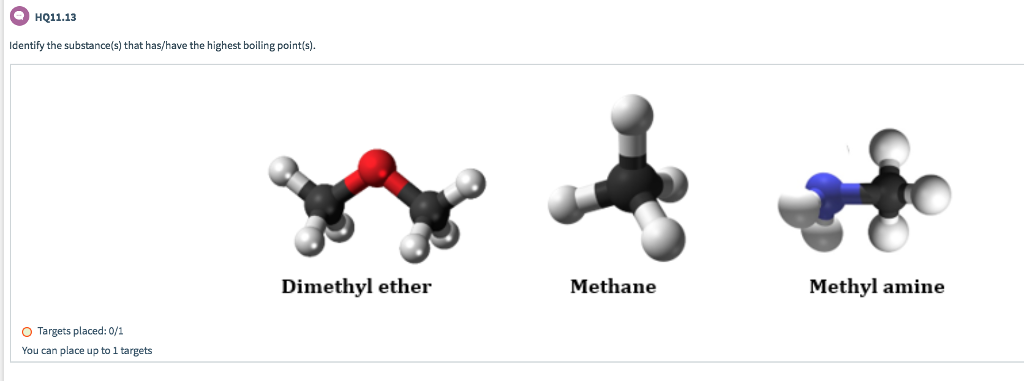
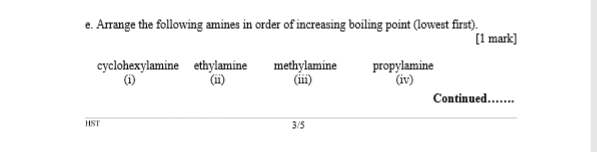
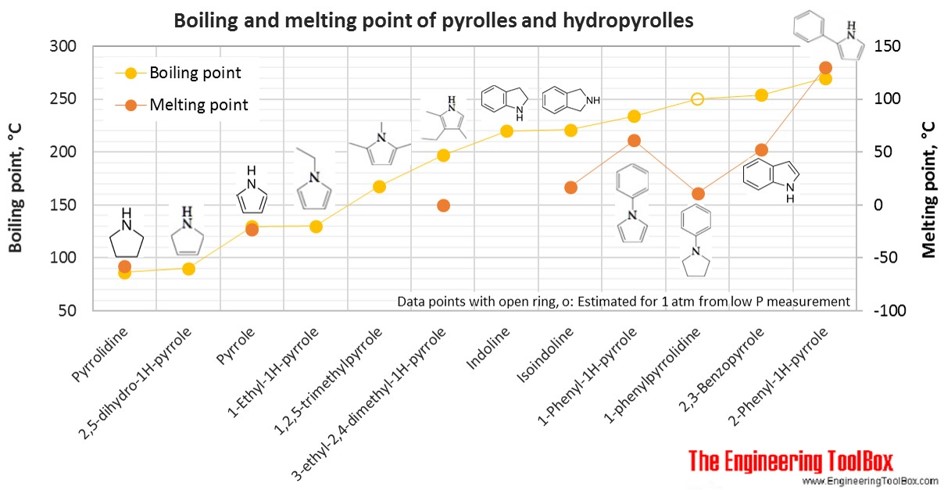
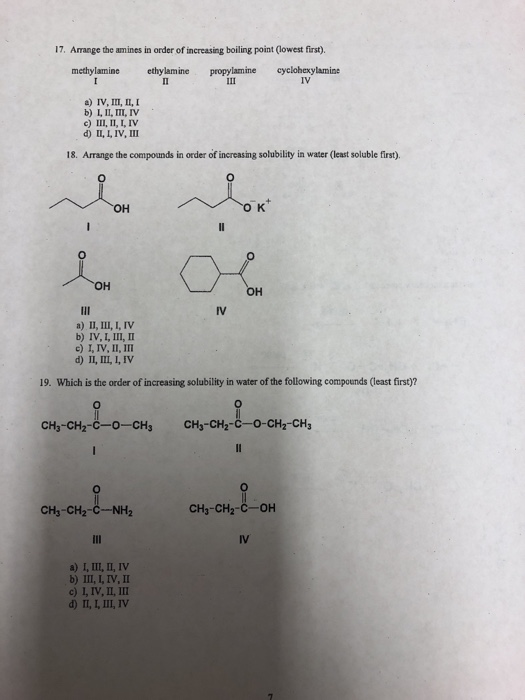
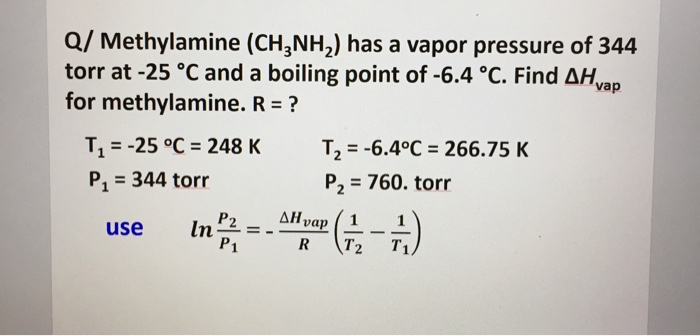
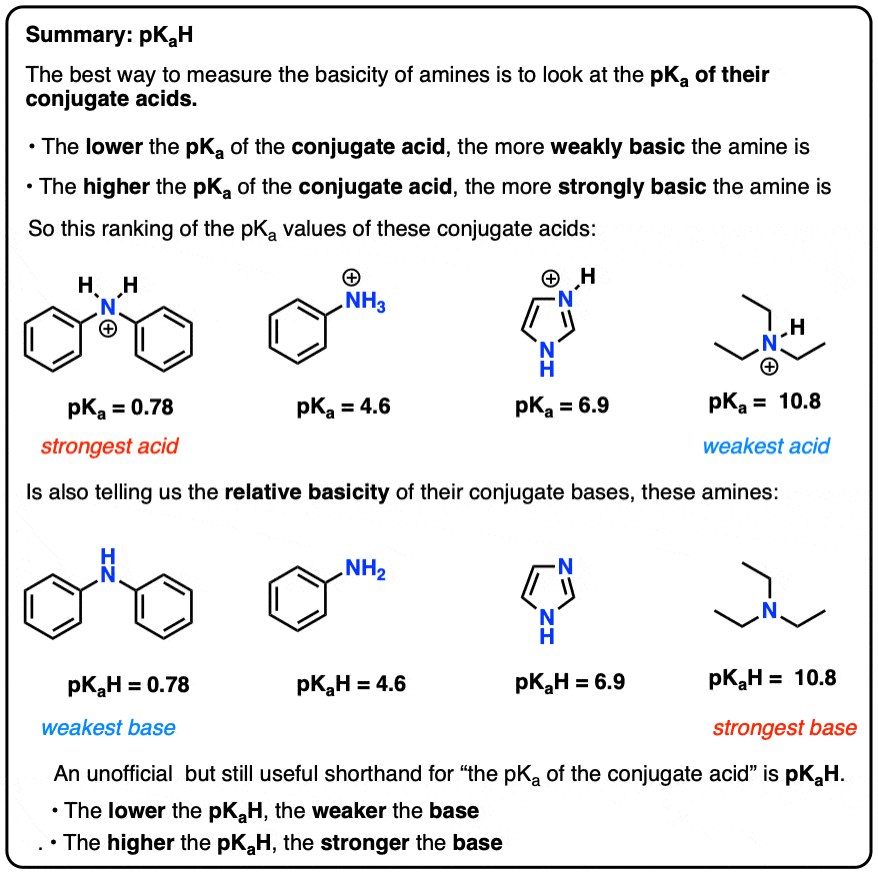
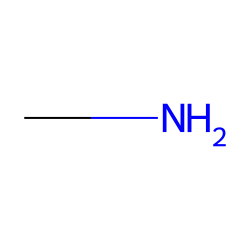
Linear aliphatic primary amines melting points boiling points solubility in water hydrogen bonding structure classification physical properties of aliphatic amines organic nitrogen compounds advanced A level organic chemistry revision notes doc brown
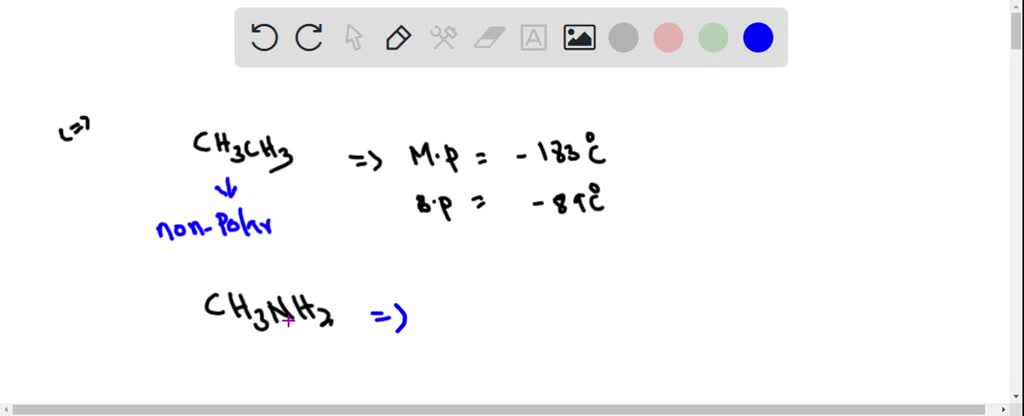
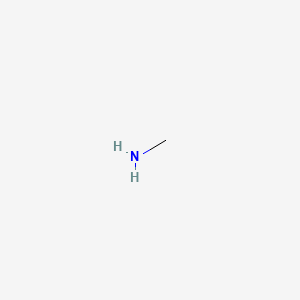
SOLVED: Ethane (CH3CH3) has a melting point of -183°Cand a boiling point of -89°C. Predict the melting point and boiling points for methylamine(CH3NH2)
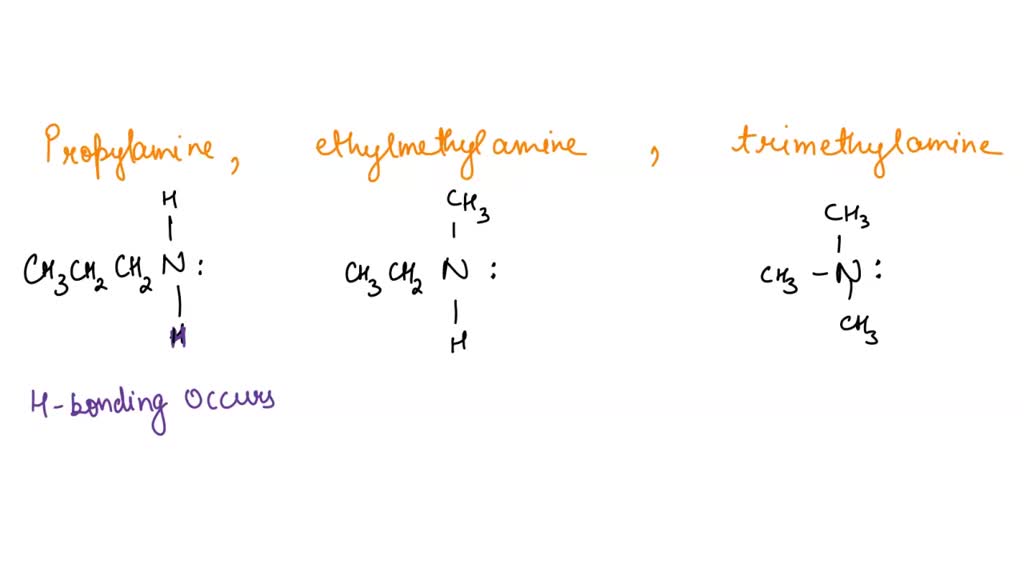
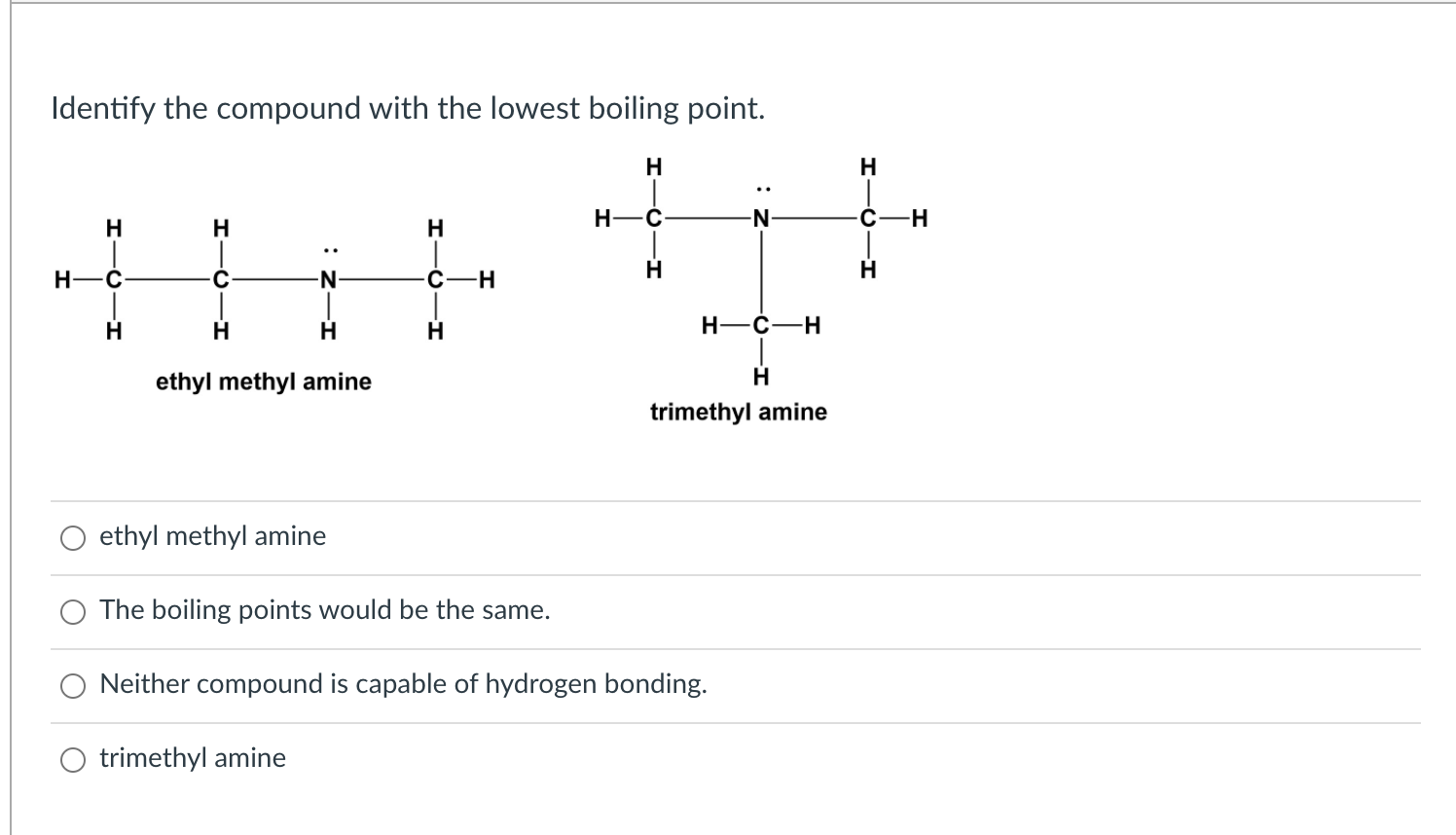
SOLVED: Q131. Propylamine, ethylmethylamine, and trimethylamine have the same molecular formula, CzHgN Explain why the boiling point of trimethylamine is considerably lower than the boiling points of the other two compounds

SOLVED: Q131. Propylamine, ethylmethylamine, and trimethylamine have the same molecular formula, CzHgN Explain why the boiling point of trimethylamine is considerably lower than the boiling points of the other two compounds