For 1 M solution of HA, the dissociation constant Ka in terms of vant Hoff factor (i) can be written as :

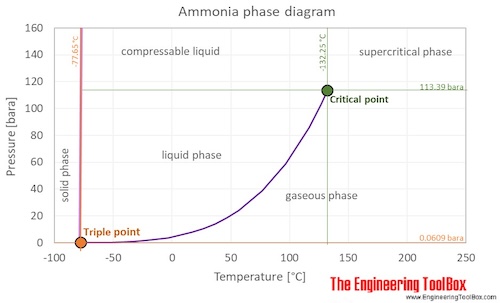
home experiment - Does the boiling point of ammonia hydroxide change with the ratio of water to ammonia? - Chemistry Stack Exchange

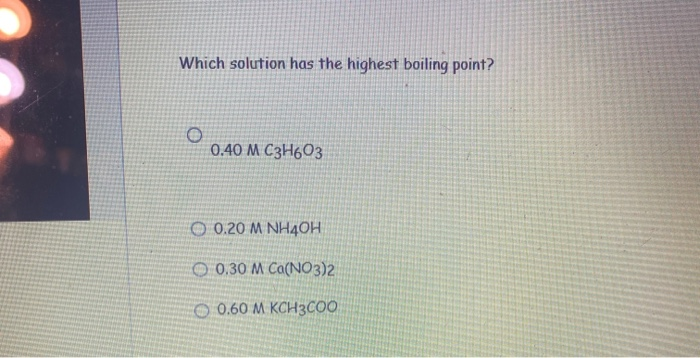
SOLVED: Which solution has the highest boiling- point? 0.40 M C3H603 0.20 M NH4OH 0.30 M Ca(NO3)2 0.60 M KCH3COO

Difference Between Ammonium Hydroxide and Sodium Hydroxide | Compare the Difference Between Similar Terms




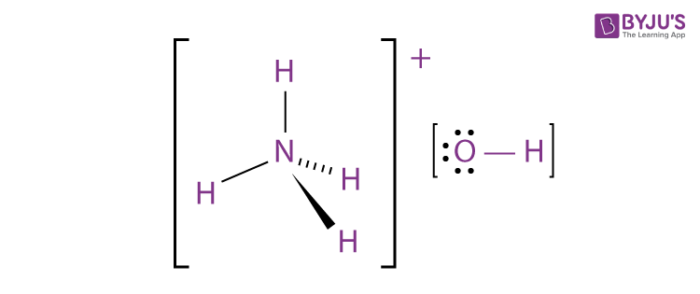



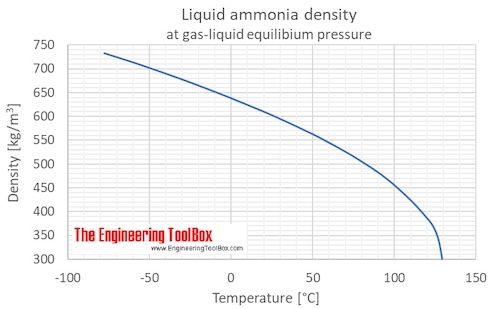

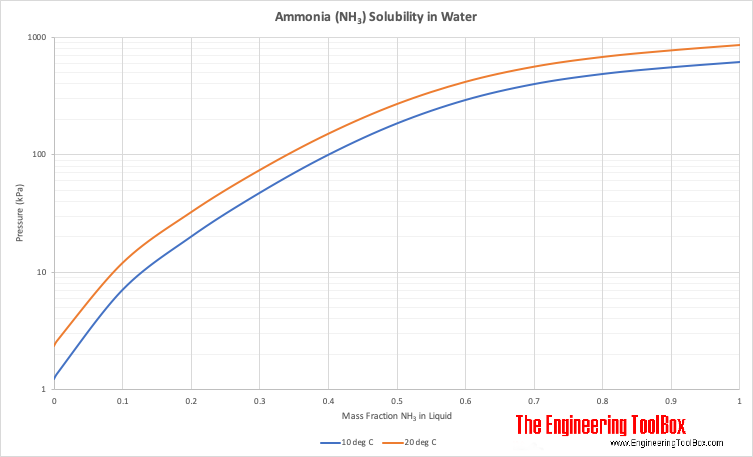





:max_bytes(150000):strip_icc()/GettyImages-143276824-5897d6d15f9b5874ee9fe6cf.jpg)