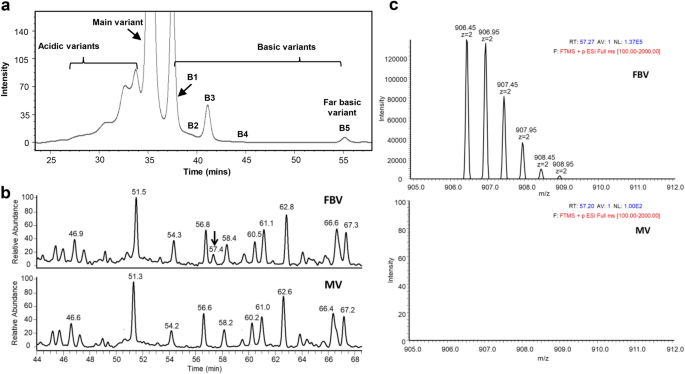
Identification, characterization and control of a sequence variant in monoclonal antibody drug product: a case study | Scientific Reports

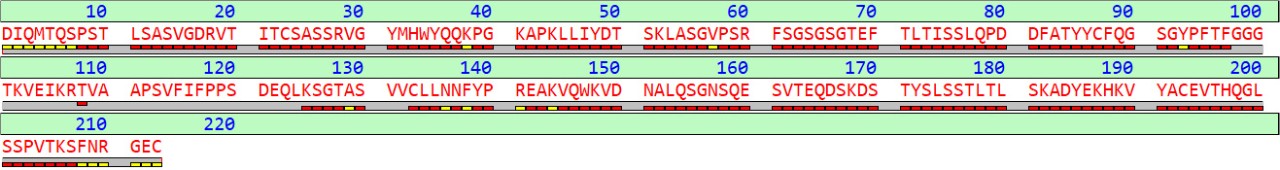
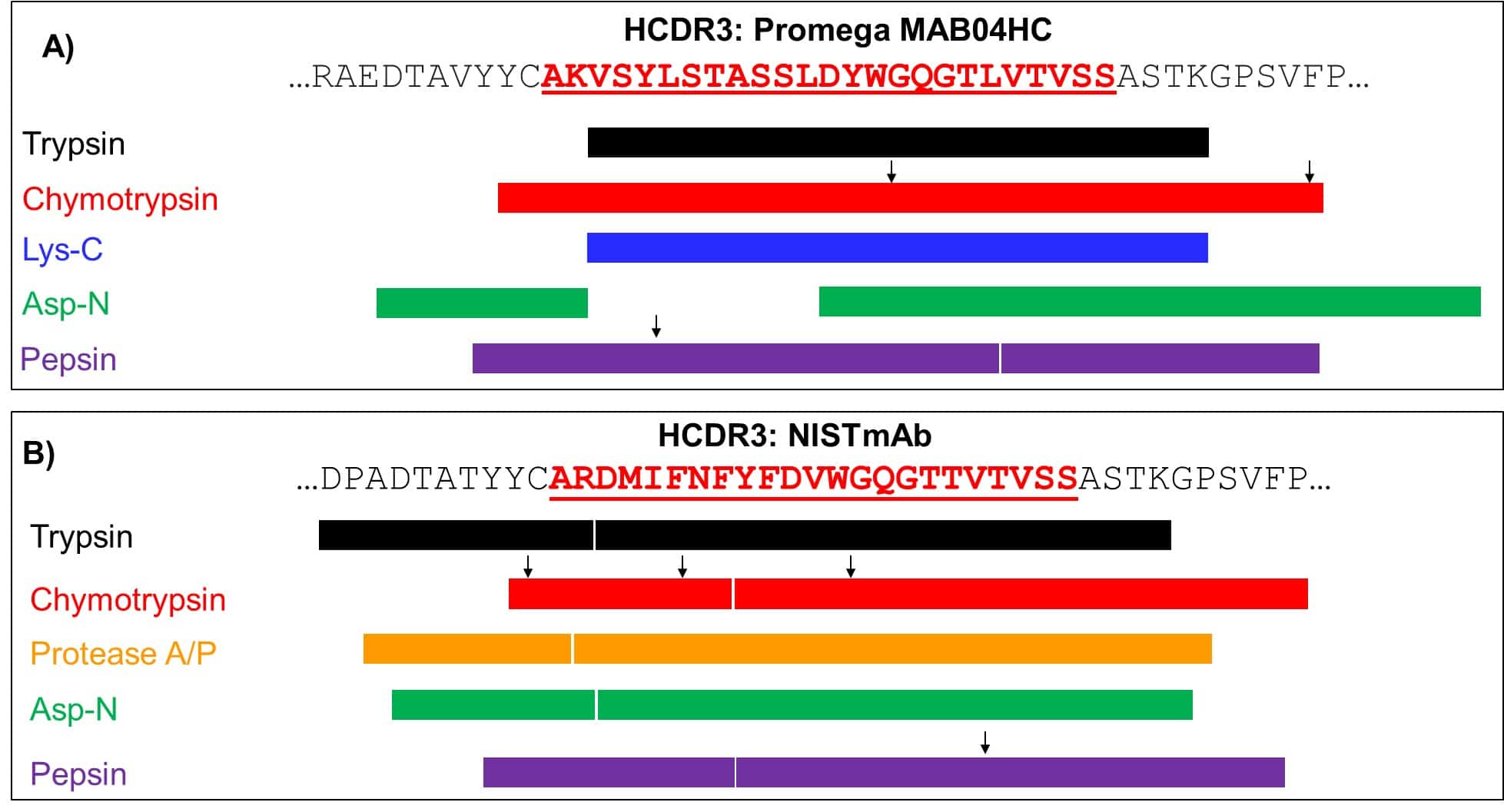
An Optimized Protocol for Peptide Mapping of Therapeutic Monoclonal Antibodies with Minimum Deamidation and Oxidation Artifacts

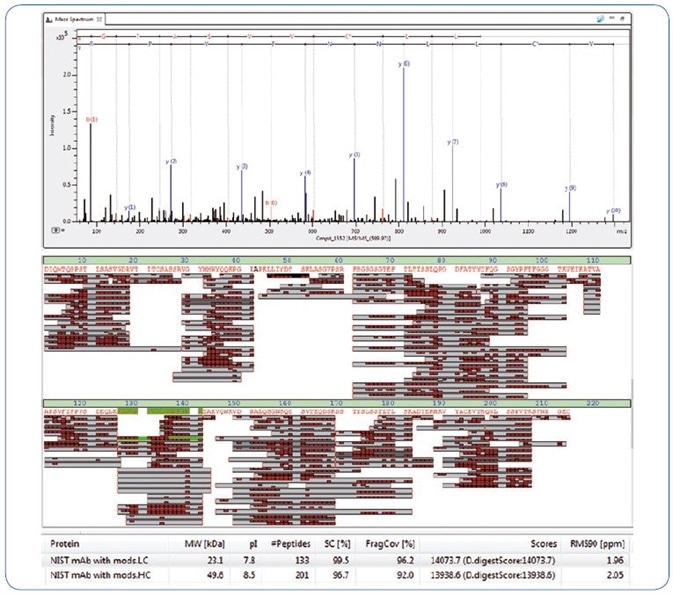
Accurate quantification of site occupancies at high sequence coverage.... | Download Scientific Diagram

Frontiers | Interlaboratory Studies Using the NISTmAb to Advance Biopharmaceutical Structural Analytics
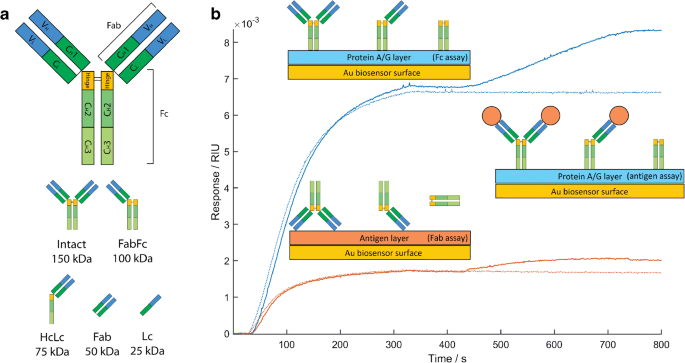
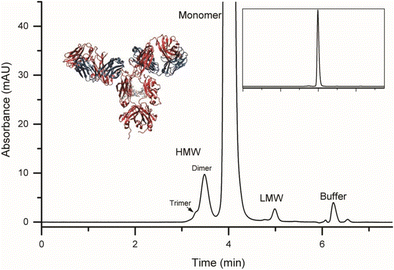
A rapid and quantitative technique for assessing IgG monomeric purity, calibrated with the NISTmAb reference material | SpringerLink
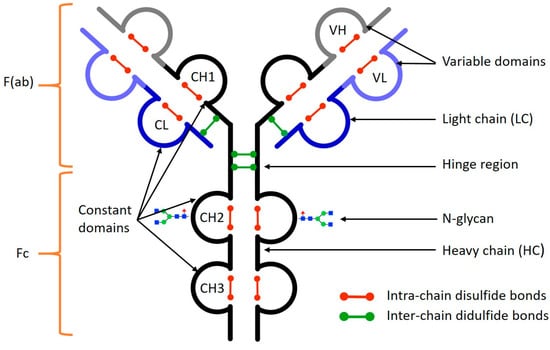
Separations | Free Full-Text | Analysis of Monoclonal Antibodies by Capillary Electrophoresis: Sample Preparation, Separation, and Detection

Structure-Specific N-Glycoproteomics Characterization of NIST Monoclonal Antibody Reference Material 8671 | Journal of Proteome Research

NIST Interlaboratory Study on Glycosylation Analysis of Monoclonal Antibodies: Comparison of Results from Diverse Analytical Methods - ScienceDirect
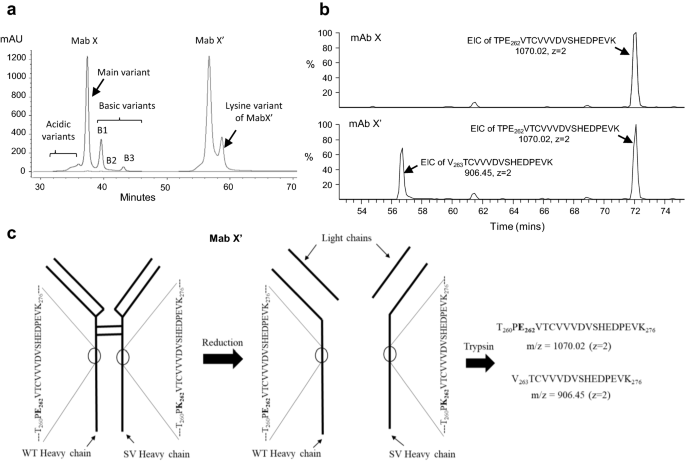
Identification, characterization and control of a sequence variant in monoclonal antibody drug product: a case study | Scientific Reports

An Optimized Protocol for Peptide Mapping of Therapeutic Monoclonal Antibodies with Minimum Deamidation and Oxidation Artifacts