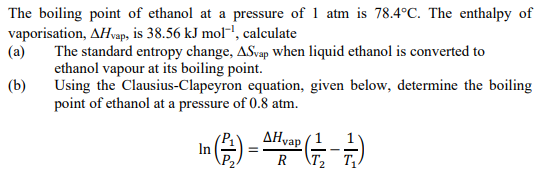
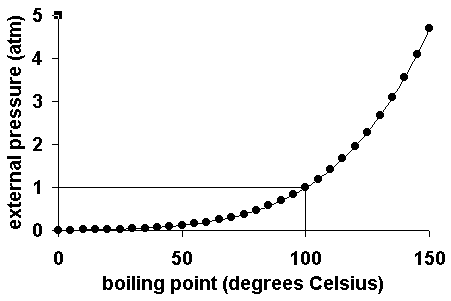
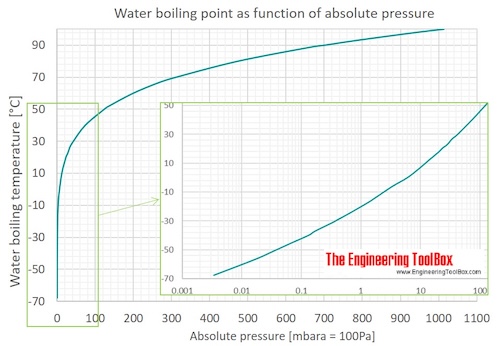
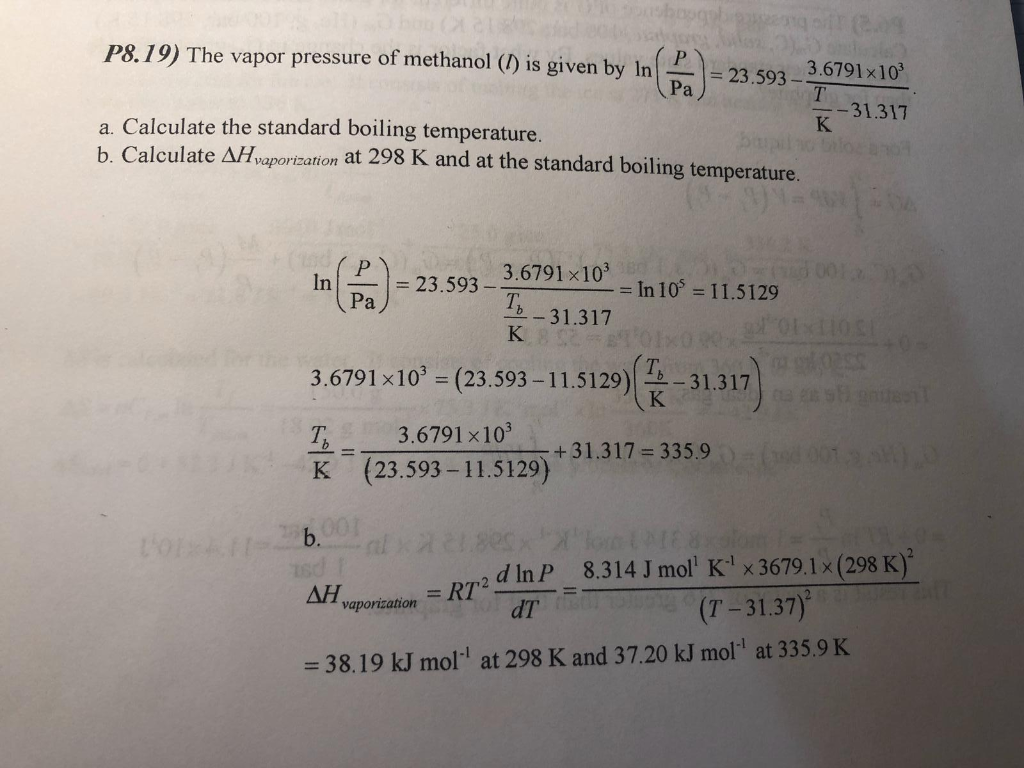SOLVED: The boiling point of methanol is 65.0^∘C and the standard enthalpy of formation of methanol vapor is -201.2 kJ / mol . Calculate the vapor pressure of methanol (in mmHg )

Given the following data, estimate the boiling point of carbon disulfide, CS2 , assuming that ?So and ?Ho are temperature-independent. Table showing the endothermic heat and the Standard entropy for | Homework.Study.com

Difference Between Normal Boiling Point and Standard Boiling Point | Compare the Difference Between Similar Terms