Chloroform Vector Illustration. Chemical Liquid Structure, Characteristics, Melting And Boiling Point Scheme. Trichloromethane Organic Compound With Formula CHCl3. Dangerous Substance For Anesthetic. Royalty Free SVG, Cliparts, Vectors, And Stock ...
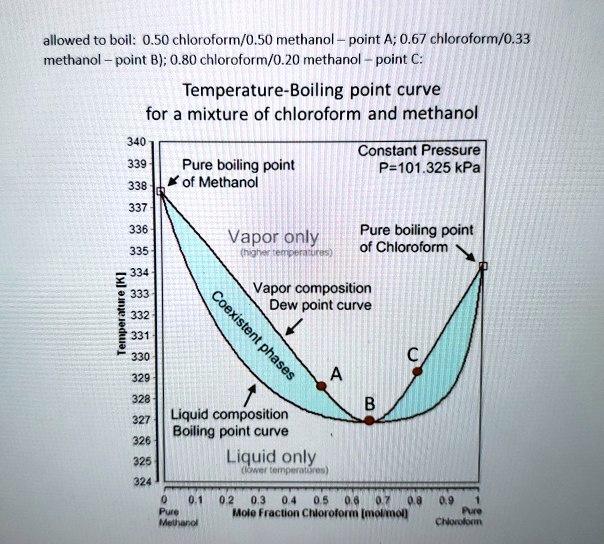
SOLVED: allowed to boil: 0.50 chloroform/0.50 methanol point A; 0.67 chloroform/0.33 methanol point BJ; 0.80 chloroform/0.20 methanol- point C: Temperature-Boiling point curve for a mixture of chloroform and methanol 340 Constant Pressure
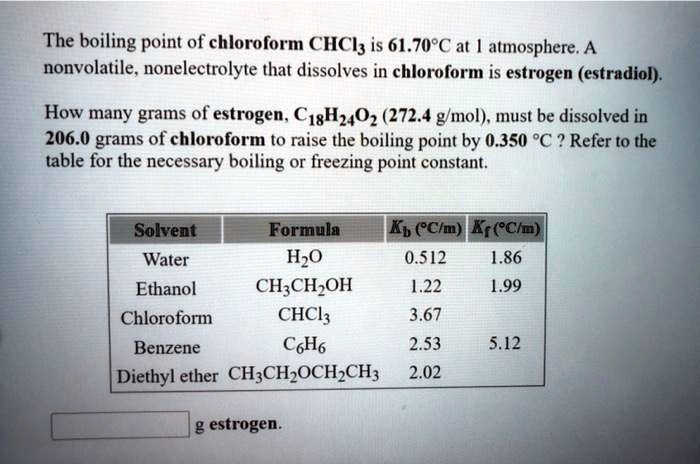
SOLVED: The boiling point = of chloroform CHCly is 61.70P€ at atmosphere. A nonvolatile, nonelectrolyte that dissolves in chloroform is estrogen (estradiol) How many grams of estrogen, C18Hz40z (272.4 g/mol) , must
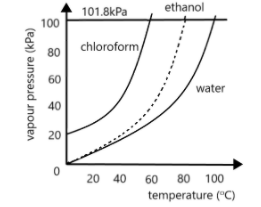
What is the normal boiling point for chloroform?\n \n \n \n \n A. $40^\\circ C$B. $50^\\circ C$C. $60^\\circ C$D. $70^\\circ C$E. $80^\\circ C$
Trichloromethane/Chloroform, 10 l, tinplate, CAS No. 67-66-3 | Solvents for Synthesis | Solvents | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International

What would be the molar mass of a compound if 6.21 g of it dissolved in 24.0 g of chloroform form a solution that has a boiling point of 68.04^o C. The

Write the correct decreasing order of boiling point for bromomethane , chloroform , dibromometha... - YouTube

Chloroform vector illustration. Chemical liquid structure, characteristics, Stock Vector, Vector And Low Budget Royalty Free Image. Pic. ESY-057771168 | agefotostock
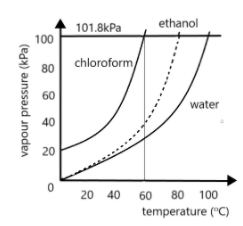
What is the normal boiling point for chloroform?\n \n \n \n \n A. $40^\\circ C$B. $50^\\circ C$C. $60^\\circ C$D. $70^\\circ C$E. $80^\\circ C$
Boiling point of chloroform was raised by 0.323 K, when 0.5143 g of anthracene was dissolved in 35 g of chloroform. Molecular mass of anthracene isKb for CHCl 3=3.9 kg mol 1

Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research

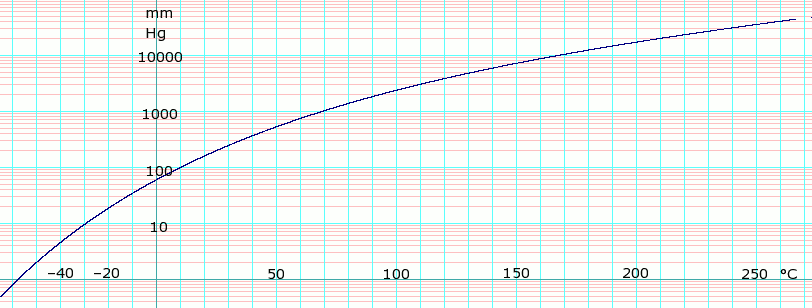
Use the figure below to determine the boiling point of -Chloroform at 80 kPa -Ethanol at 20kPa -Ethanol at - Brainly.com